
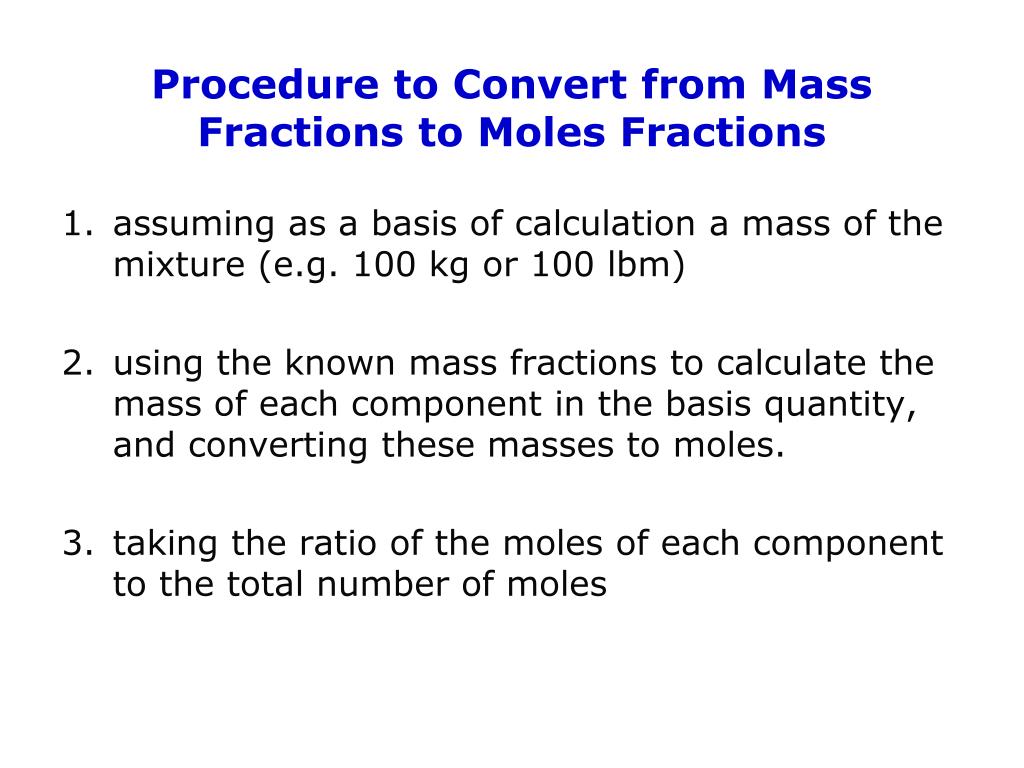
The two decimal point atomic weights for Nickel and Chromium are: The molar mass of Cl2 (which we get from the atomic mass of Cl from the. %:Īs an example, let’s take a standard (Ni)80(Cr)20 Wt. We can add that conversion factor as another step in a calculation to make a. % of x as determined above from 100%.Ĭonversely to convert from Wt. % of y in the same manner by recalculating after substituting all the x’s and y’s or by simply subtracting the Wt. For example, given two elemental constituents, lets just call them “x” and “y”, we can convert from At.% to Wt. By looking up the atomic or molecular weights of the individual element or molecular constituents to as many significant figures as desired, it is possible to make the simple conversions from one to the other. This can be done quite easily by using the atomic weight (At. Therefore, it is necessary to convert from At. So it is possible construct alloys and compounds using individual atom counts, but if you don’t want to deal in numbers in the 10 to the 23rd power range it is a lot easier to deal with atomic weights or molecular weights. Using a measurement referred as a mole we can use Avogadro’s number and atomic mass units and molar mass units and the like to determine the actual number of atoms there are in a gram of a particular material.
#Mass fraction to atomic fraction converter how to
We have all learned how to figure out how many atoms of a particular element are in a gram back in school. It is a lot easier to weigh out the individual constituents in grams, or the like, rather than in atoms. %) when manufacturing an alloy or composite material. Therefore, it is necessary for us to deal in weight percentages (Wt. However, for actual manufacturing we cannot measure individual atoms very easily. when an optical, chemical, electrical, physical, etc.

This is the area where we at Plasmaterials are often dealing.Ītomic percentages are all well and good when the concern is how individual atoms from one species or another provides a solution to a problem according to how they interact on an atomic level, i.e. The exception is within the scientific community where atomic percentages are the norm. Unless otherwise stated, liquids and solids are generally expressed in weight percentages and gasses are expressed in volume (Vol. Accordingly, we generally receive requests for quotations in atomic percentages or molecular percentages (Mol. %) level when considering such properties. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass. So, in general, they deal on an Atomic Percentage (At. The sum of all the mass fractions is equal to 1. Therefore, they generally think along the lines of atomic interactions from one element to another, how a certain stoichiometric property alters as the composition is changed or an additive is introduced. Such customers are more often concerned with material properties on an atomic level when dealing with elemental materials, alloys and composites.

are scientists, engineers, educators or people working in some form of research and development, be it either in academia or industrial oriented. In a spatially non-uniform mixture, the mass fraction gradient gives rise to the phenomenon of diffusion.A good portion of our customer base here at Plasmaterials, Inc. In chemistry, the mass fraction of a substance within a mixture is the ratio w i Spatial variation and gradient Fraction of one substance's mass to the mass of the total mixture


 0 kommentar(er)
0 kommentar(er)
